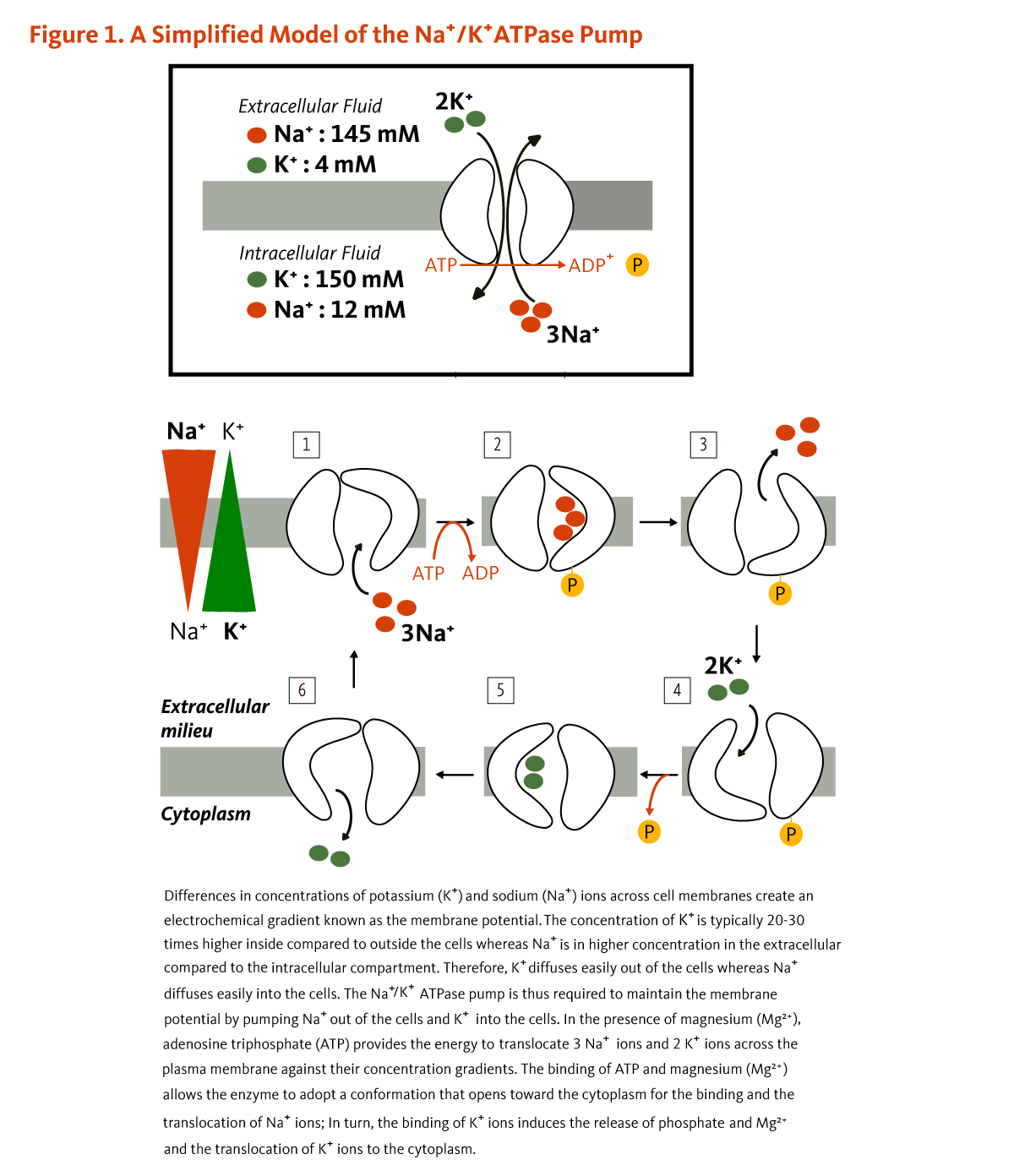
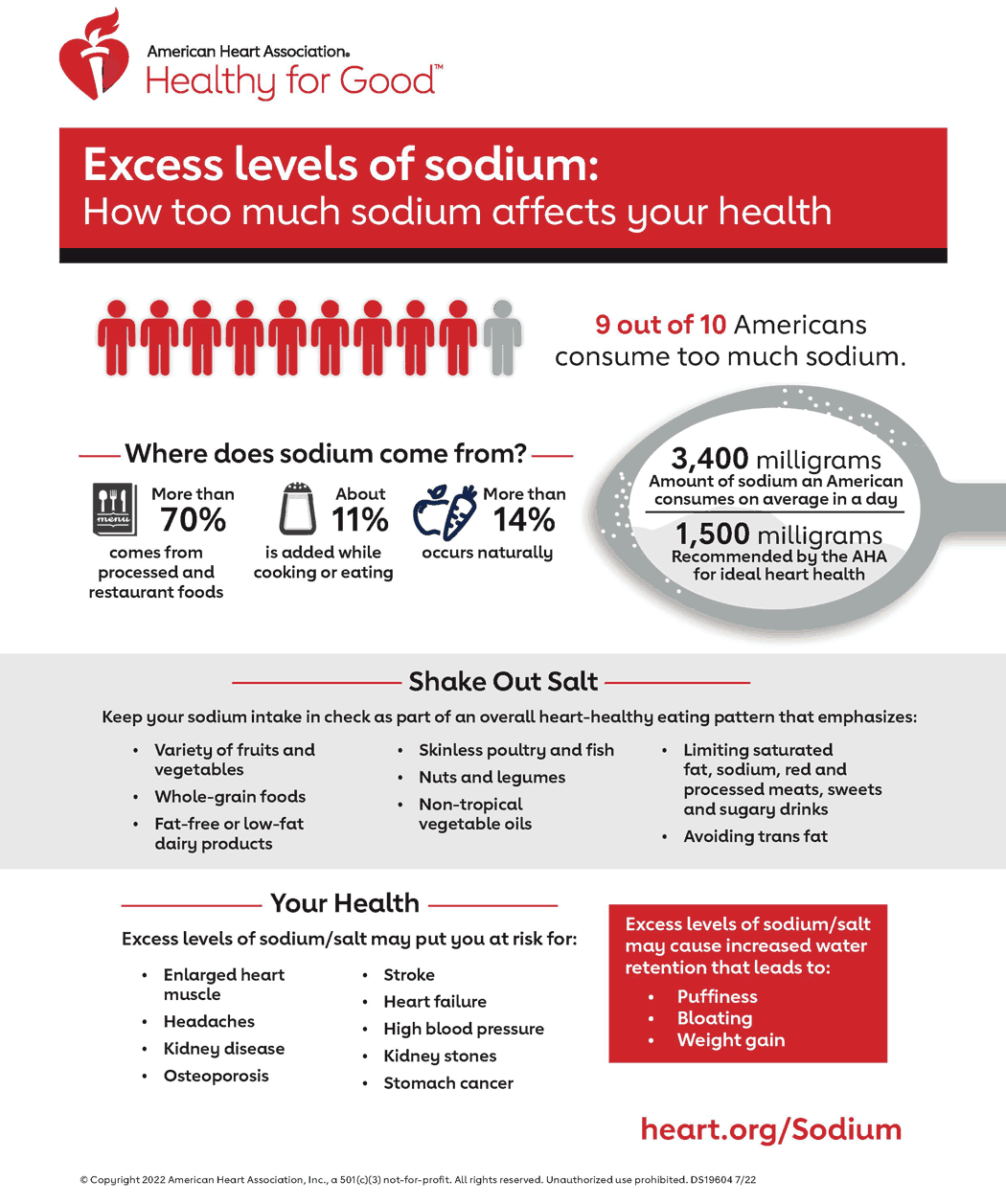
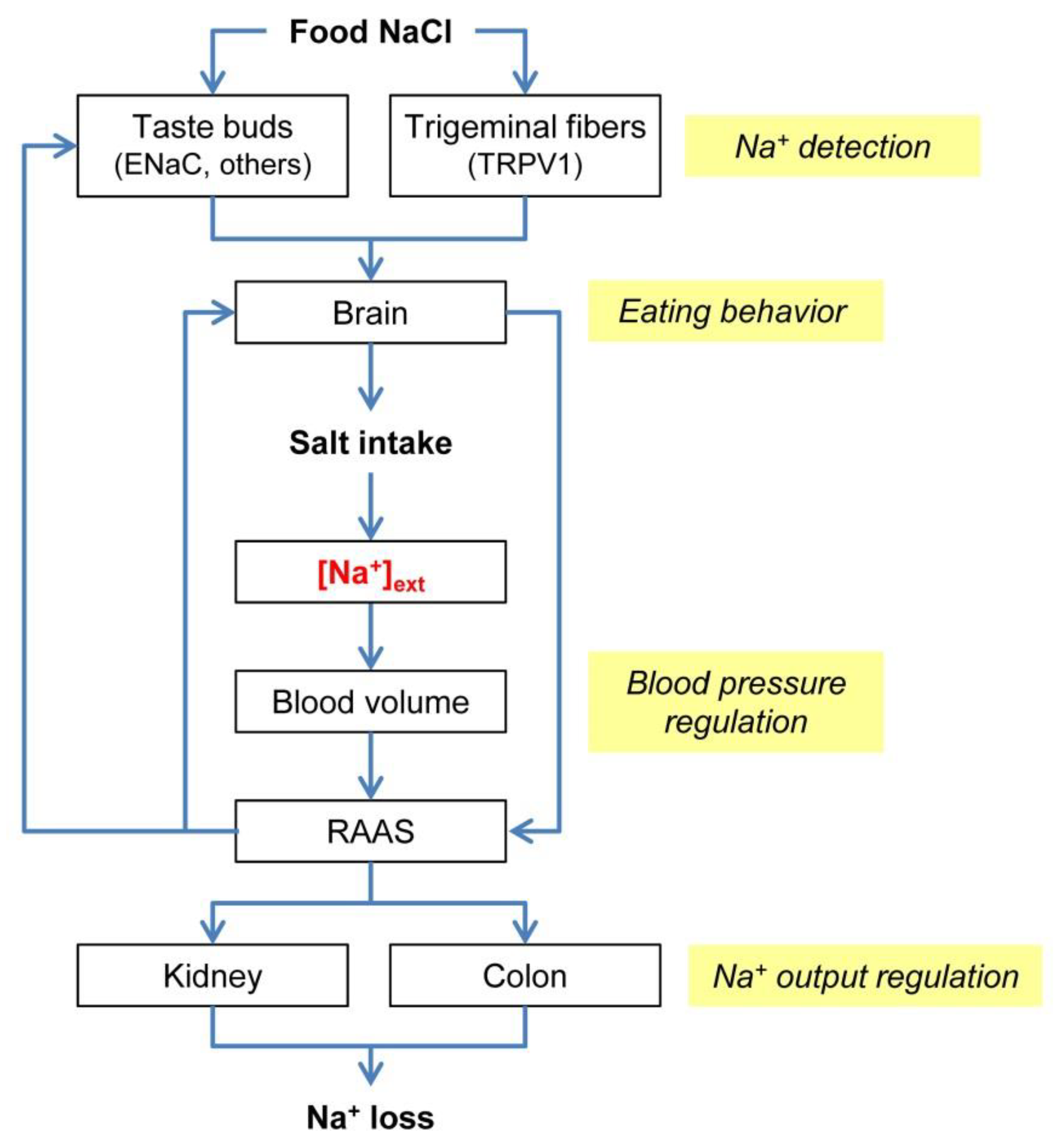
Effect of dose and duration of reduction in dietary sodium on blood pressure levels: systematic review and meta-analysis of randomised trials | The BMJ
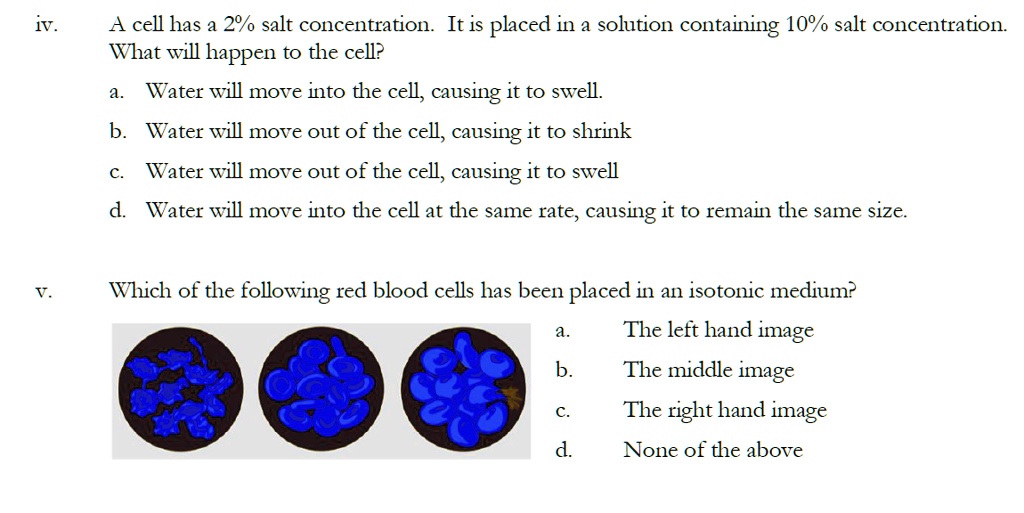
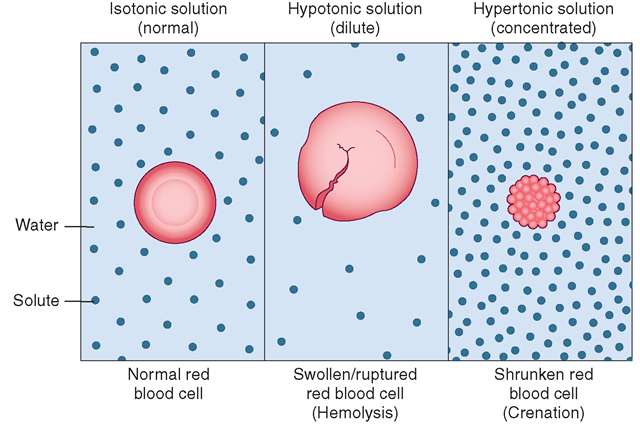
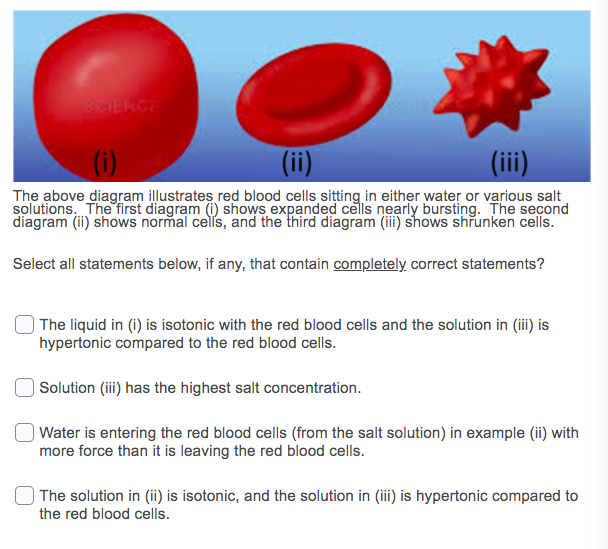
SOLVED: A cell has a 2%/ salt concentration: It is placed in a solution containing 10% salt concentration What will happen to the cell? Water will move into the cell, causing it