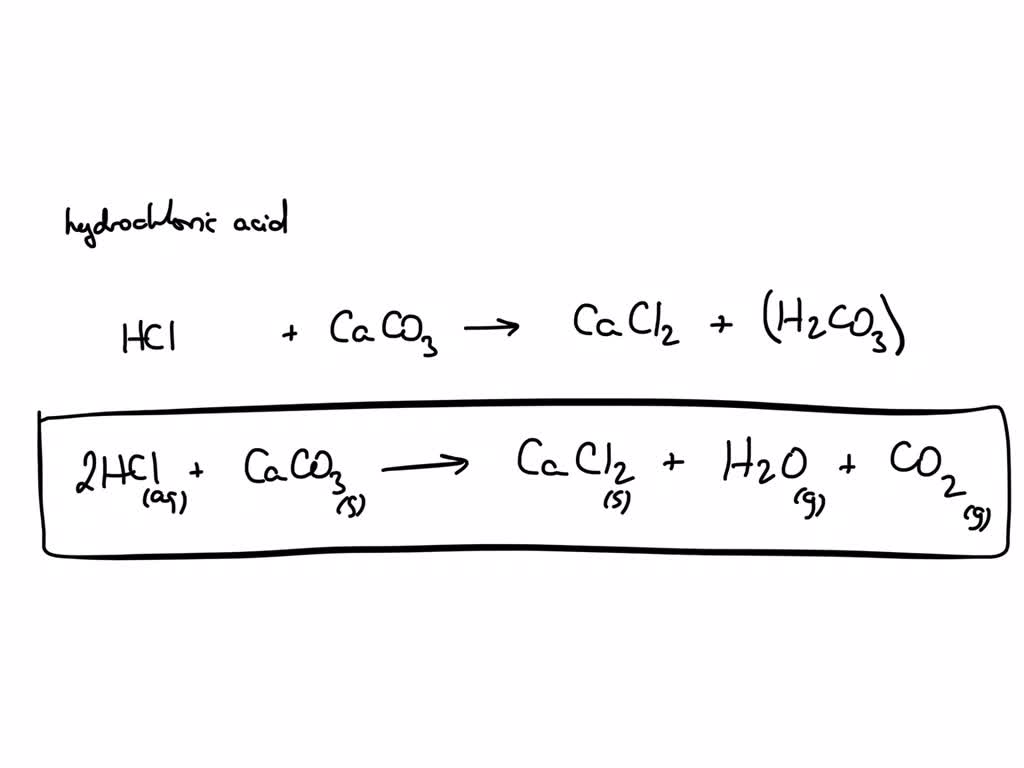
Balance the following equations : (a) Caco, (s) + HCl (aq) + CaCl, (aq) + H2O (1) + CO, (g) (b) Zn (s) + HCl - Brainly.in
The mass of CaCO3 required to react completely with 20 mL of 1.0 M HCL as per the reaction CaCO3+2HCl–>CaCl2+CO2+H2O
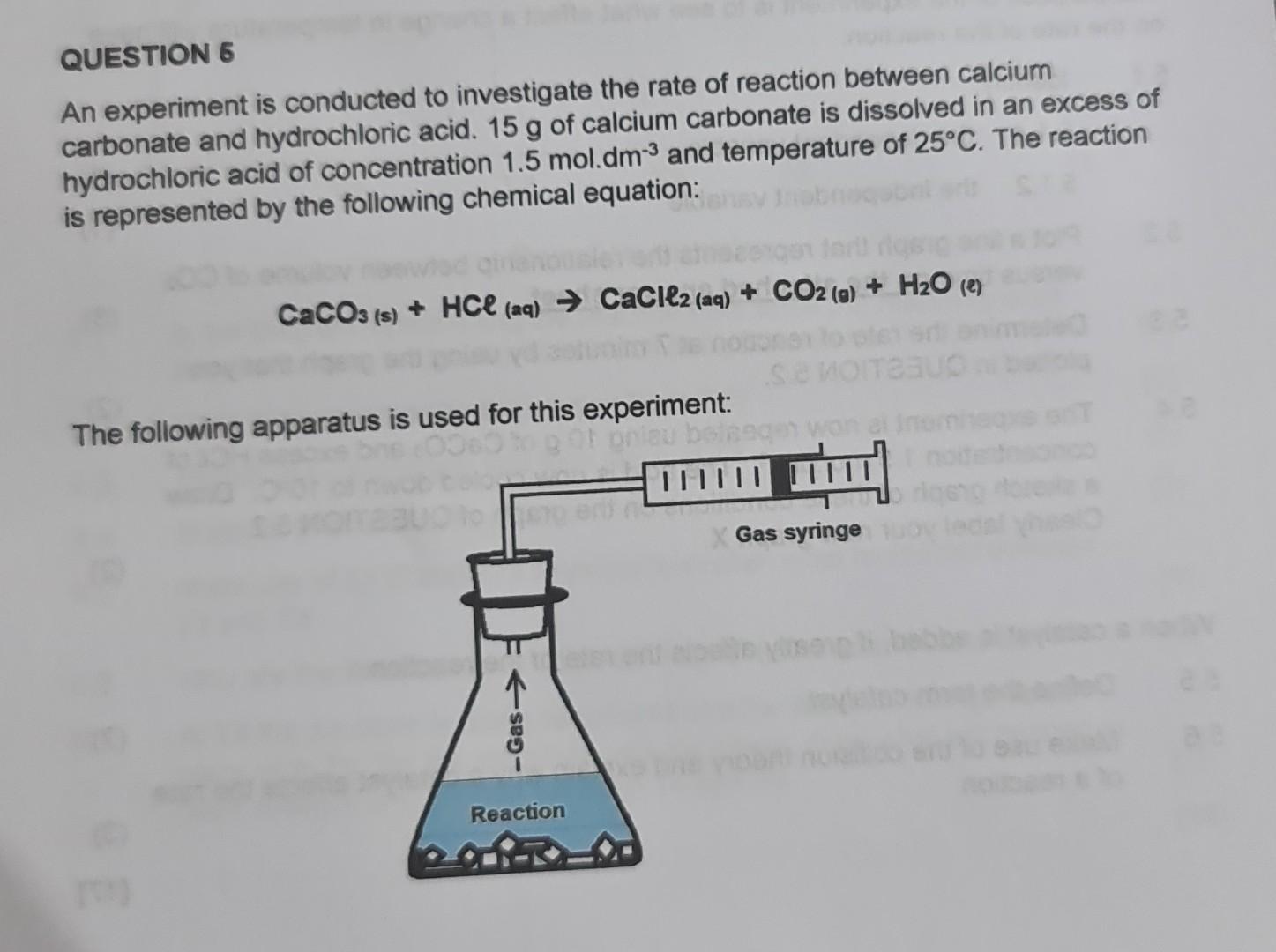
35. a) Calcium carbonate reacts with aqueous HCl according to the reaction: CaCO_3(s)+2HCl(aq)→ CaCl_2(aq)+CO_2(g)+H_2O(l) . What mass of CaCO3 is required to react completely with 25ml of 0.75M HCl b) 1.0g of
30. Calcium carbonate reacts with HCl to give CaCl,and CO2 according to the reaction:+ 2HCI(aq) CaCl, (aq) + CO2(g)CaCO3(s)H20(I) What mass of 20
calcium carbonate reacts with aqueous HCL to give cacl2 and CO2 according to the reaction caco3 + HCL gives cacl2 + CO2 + H2O what mass of caco3 is required to completely

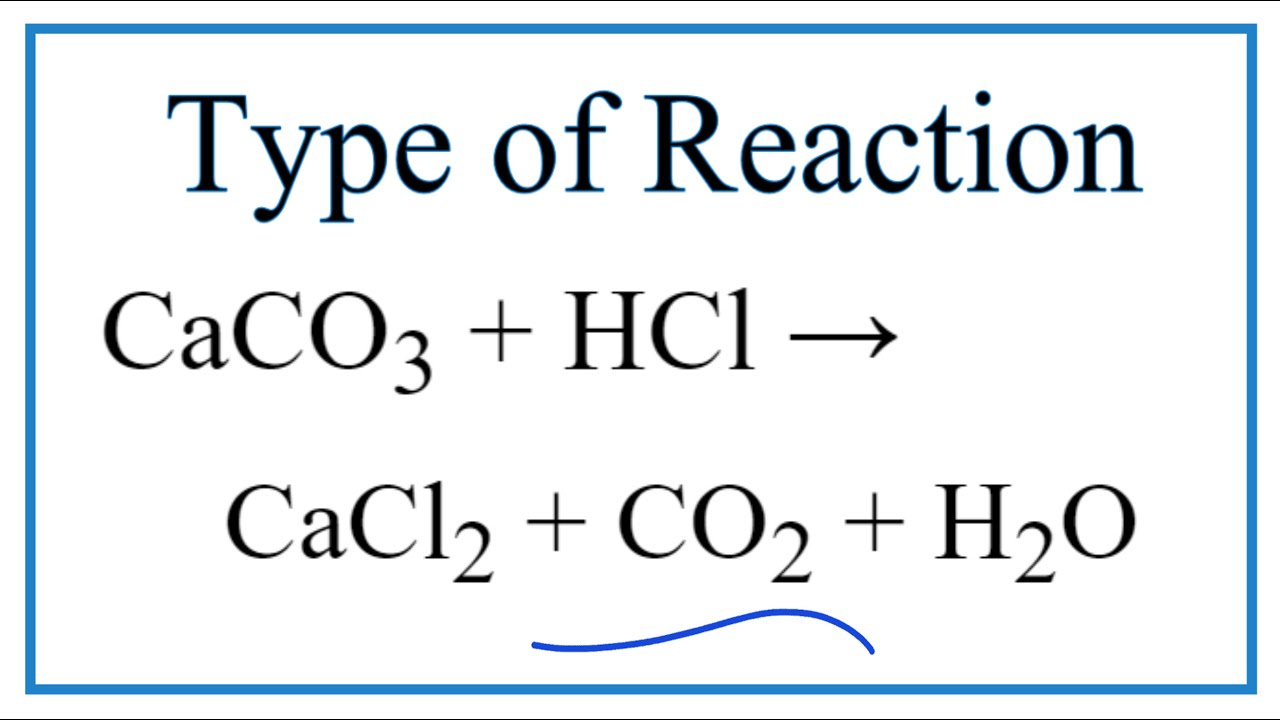
Predict the Products of the Reaction for CaCO3 + HCl (Calcium carbonate + Hydrochloric acid) - YouTube

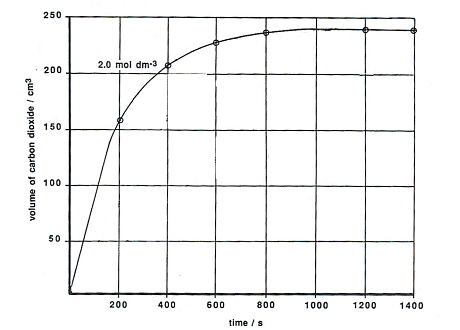
Final conversion rates of CaCO3 , modified CaCO3 and Ca( OH) 2( HCl... | Download Scientific Diagram